Drainless Tummy Tuck with TissuGlu
TissuGlu® Surgical Adhesive is used internally during abdominoplasties (tummy tucks). Once applied, TissuGlu strongly bonds the tissue layers together. This bond reduces the space between the layers where fluid may collect. After the layers heal, TissuGlu breaks down into safe components that are then eliminated by the body naturally.
Ask Dr. Ennis for further information about TissuGlu Surgical Adhesive including the full contraindications, warnings, precautions, and risks and benefits.
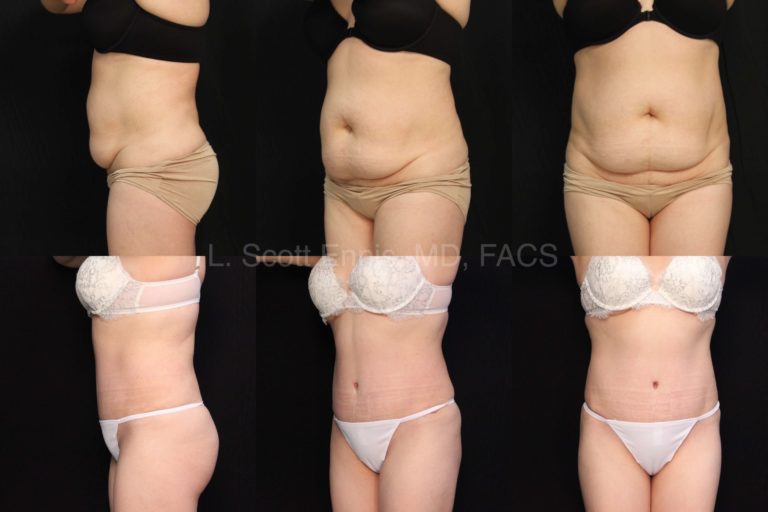
Actual Drainless Tummy Tuck Patient Photo of Dr. Ennis
TissuGlu Video
Important TissuGlu® Surgical Adhesive Safety Information
INDICATION
TissuGlu® Surgical Adhesive is indicated for the approximation of tissue layers where subcutaneous dead space exists between the tissue plane in abdominoplasty.
IMPORTANT SAFETY INFORMATION
Contraindications
Do not use in patients with known or suspected allergies to urethane-based or isocyanate-containing products.
Warnings
Do not use TissuGlu® Surgical Adhesive in patients who have had prior exposure to TissuGlu®. Immunological response associated with repeat TissuGlu® exposure has not been studied.
The effectiveness for the treatment of patients with BMI > 28 has not been established. Higher BMI patients have a propensity for fluid accumulation and may have an increased risk of seroma formation.
Effectiveness was not observed in weight loss patients undergoing abdominoplasty. Weight loss patients have a propensity for fluid accumulation and may have an increased risk of seroma formation and aspiration.
Benefits
TissuGlu® may reduce the need for drains after surgery. By using TissuGlu® instead of drains, you may:
Return to normal activities more quickly
Experience less discomfort and pain than when drains are used
Have fewer invasive treatments for fluid management. The results showed that 73% of TissuGlu® patients had no treatments after surgery. However, some patients did require additional invasive treatments including reinsertion of drains.
Risks
Clinical studies were performed using TissuGlu®. There were no increased safety risks with the use of TissuGlu® than when drains were used. Potential complications associated with the use of the device and the tummy tuck procedures include seroma (fluid) formation. These complications may require treatments such as drain placement and/or fluid removal by needle, which may be performed in the operating room. Additional potential complications include wound healing issues, rash/redness, surgical site or incision infection, scarring, hematoma (bruise or swelling), incision separation, and immunological reaction.
Safety Information:
For full safety information please visit http://tissuglu.com, contact your doctor, or call Dr. Ennis at 866-364-2623.
Disclaimer:
TissuGlu® Surgical Adhesive is currently approved for sale in the United States for the approximation of tissue layers where subcutaneous dead space exists between the tissue planes in abdominoplasty. TissuGlu® Surgical Adhesive received European CE Mark in 2011, where it is approved for large flap surgical procedures such as abdominoplasty. TissuGlu® Surgical Adhesive is not approved for sale in any other region. Any questions related to the indication for use should be directed to your Cohera Medical Account Manager or to Cohera Medical Corporate Clinical Affairs. Refer to the complete TissuGlu® Surgical Adhesive Directions for Use for prescribing and usage information.
